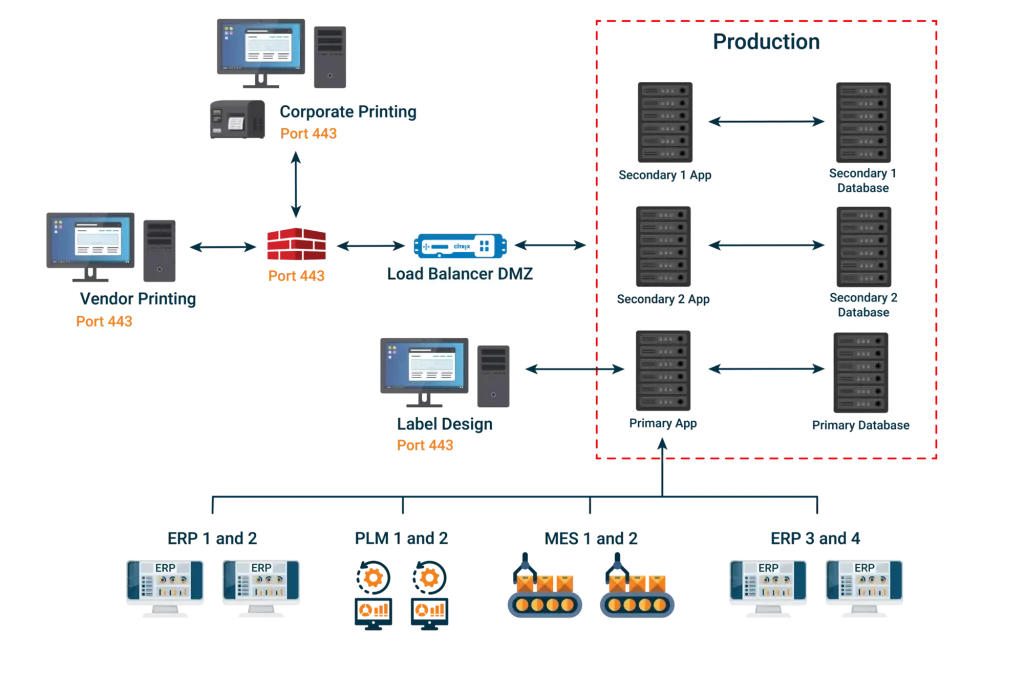
Labeling System Architecture

Make Sure That Your System Architecture is Ironclad And Future Proof
ROBAR’s Enterprise Architecture Enables Robust Labeling, Submissions, and Regulatory Data Management for Any Life Sciences Manufacturer.
Select Customers

























A Better Architectural Design
Understand Your Definition
The architecture of a life science labeling system for the life sciences strongly determines the potential trajectory for enterprise labeling, regulatory data management, and submissions capabilities of any life sciences manufacturer. The first step in detailing necessary system architecture attributes is defining exactly what enterprise labeling entails for any given life sciences manufacturer.
Some Common System Architecture Challenges for Life Sciences Manufacturers
· Deciding between on-premise and hosted
· Efficiently and securely enabling 3PLs and CMOs
· Maintaining synchronization between regulatory submissions and labeling data
· Tying together workflow approval, change control, and production
· Maintaining compliance within a capable system design
Addressing System Architecture Challenges
Mark Sikorski, Director Sales and Marketing, Software Solutions states that the first step to reaching your desired state is to understand your present state.
“Once you understand what you want and what you are missing, you can develop a plan to make things the way you want them to be.”
The problems of deciding between on-premise and hosted is much easier to resolve by adopting an architecture that allows you to easily move between the two. Enabling 3PLs and CMOs with a centrally managed, browser-based label system allows your company to scale in a controlled and realistic way through the Internet. Using ROBAR’s Master Data Management system for labeling data addresses overlaps between submissions and labeling thereby minimizing complexity and risk. An architecture that bakes in approvals and change control on your path to production ensures efficiency and compliance
Superior Software and Extraordinary Support
One thing is certain within the life sciences manufacturing industry; regulatory challenges will continue to compound. It is impossible to foresee all new requirements in advance. But, by adopting a configurable system that adapts to new requirements in real time through flexible architecture, it is possible to continually build a better way of working. As Ardi Batmanghelidj, Innovatum’s President and CEO asserts:
“By driving design elements based upon a wide breadth of potential future requirements, it is entirely achievable for us to maintain an underlying system architecture that can grow with any manufacturer in the life sciences industry.”
Exploiting a Better System Architecture Design
To exploit a system that is capable of satisfying needs today and adapting to new requirements in the future, a company must first adapt an architecture that is designed to handle growth in requirements and capabilities. As legislation changes, new market opportunities arise, reducing challenges and improving time to market is key to maintaining competitiveness in an ever-changing landscape
We Work With Organizations Around the World
We provide complete systems, modular systems, consulting, training, and exemplary 24/7 support services across all borders and time zones.